Rationale
Exploring the Potential of Dry Powder Inhalation of Aspirin for Non-Small-Cell Lung Cancer (NSCLC) Treatment
Epidemiological and non-clinical studies suggest aspirin reduces lung cancer risk and directly inhibits cancer cell growth, highlighting the potential benefits of localized, topical delivery via inhalation over oral administration.
Preclinical studies show that inhaled chemotherapeutics demonstrate significantly higher lung bioavailability compared to intravenous delivery, making inhalation a promising method for maximizing aspirin’s effectiveness against lung cancer.
Non-clinical studies indicate that aspirin directly acts on lung cancer cells, inhibiting their growth, suggesting that topical delivery through inhalation may be more effective than oral administration.
Oral aspirin has already been linked to improved survival rates in cancer patients, and studies using animal models indicate that inhaled chemotherapeutics could further enhance survival outcomes in lung cancer therapy.
NOVEL Approach
Dry Powder Inhalation of Aspirin
BIOINHALE R&D finding delivers aspirin directly to deep lung sites through Dry Powder Inhalation, a novel method for treating lung cancer. This approach is considered safe for most patients, based on prior toxicology and safety studies.
BIOINHALE Dry Powder Inhalation minimizes delivery of aspirin targets delivery to the lungs and elsewhere—thus limiting adverse effects including gastrointestinal issues—while increasing serum concentrations at the level of the lung tumor. These increased concentrations at the tumor site augment the clinical benefits of the aspirin. Additionally, inhalation of the drug bypasses inactivation by hepatic enzymes.
Several mechanistic studies have supported the use of aspirin as a potential chemotherapeutic agent in cancer treatment. Aspirin leverages both COX and non-COX pathways to inhibit tumor growth and development.
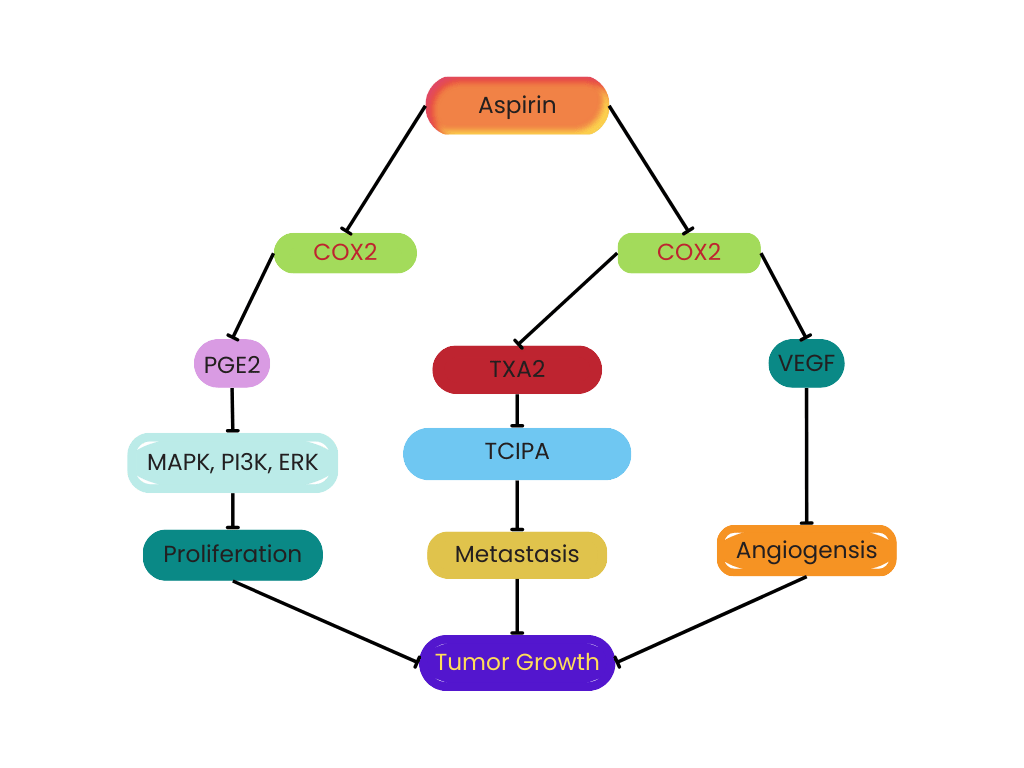
Aspirin suppresses tumor growth via the COX pathways in various ways. First, aspirin inhibits COX-2 to reduce PGE2 levels; PGE2 promotes tumor cell proliferation through multiple signaling pathways, including MAPK, PI3K, and ERK. Second, aspirin leads to platelet dysfunction and hinders platelet release by inhibiting COX-1, which can promote tumor metastasis by inducing TXA2 and TCIPA. Third, aspirin can inhibit COX-1 in platelets, yielding decreased levels of serum VEGF, thus inhibiting tumor angiogenesis. The non-COX pathway anti-tumor mechanisms of aspirin could inhibit tumor-related transcription factors, including the activation of transcription factor-1 (AP-1) and nuclear factor kappa B (NF-ƘB).
One recent study showed that aspirin inhibited lung cancer stemness and exosome function in a hypoxic tumor microenvironment—similar to that of lung cancer. Another recent study showed that aspirin targeted the mir-98/WNT1 axis, with cell viability sharply hampered in lung cancer cells exposed to increasing aspirin concentrations. Moreover, in this study, aspirin depleted the malignant colony formation ability of lung cancer cells.
Clinical research has supported the use of aspirin as chemotherapy in patients with lung cancer. For instance, low-dose aspirin in the year prior to diagnosis was found to be associated with lower tumor extent and fewer metastases in patients with colorectal and lung cancers. Furthermore, a recent meta-analysis that included lung cancer patients demonstrated that patients taking aspirin at pre- and post-diagnosis experienced a significantly decreased risk of distant metastases.
Clinical research has supported the use of aspirin as chemotherapy in patients with lung cancer. For instance, low-dose aspirin in the year prior to diagnosis was found to be associated with lower tumor extent and fewer metastases in patients with colorectal and lung cancers. Furthermore, a recent meta-analysis that included lung cancer patients demonstrated that patients taking aspirin at pre- and post-diagnosis experienced a significantly decreased risk of distant metastases.
“Association of Long-term Use of Low-Dose Aspirin as Chemoprevention with Risk of Lung Cancer,” by Ye et al, JAMA (2019)
a. Long term low-dose aspirin use reduced the incidence of lung cancer by 11% in a high-power study which included 63,040 subjects.
“Regular Adult Aspirin Use Decreases the Risk of Non-Small Cell Lung Cancer Among Women”, by Van Dyke et al., Cancer Epidemiology Biomarkers Prevention (2008) http://cebp.aacrjournals.org/content /17/1/148.
- “The Anti-Tumor Effect of Aspirin: What We Know and What We Expect” Review by Ma et al. Biomedicine and Pharmacotherapy (2017)
- Aspirin inhibits COX1 and COX2 enzymes inhibiting tumor growth.
Figure 1. Tumor Growth Through COX1 and COX2 Dependent Pathway.
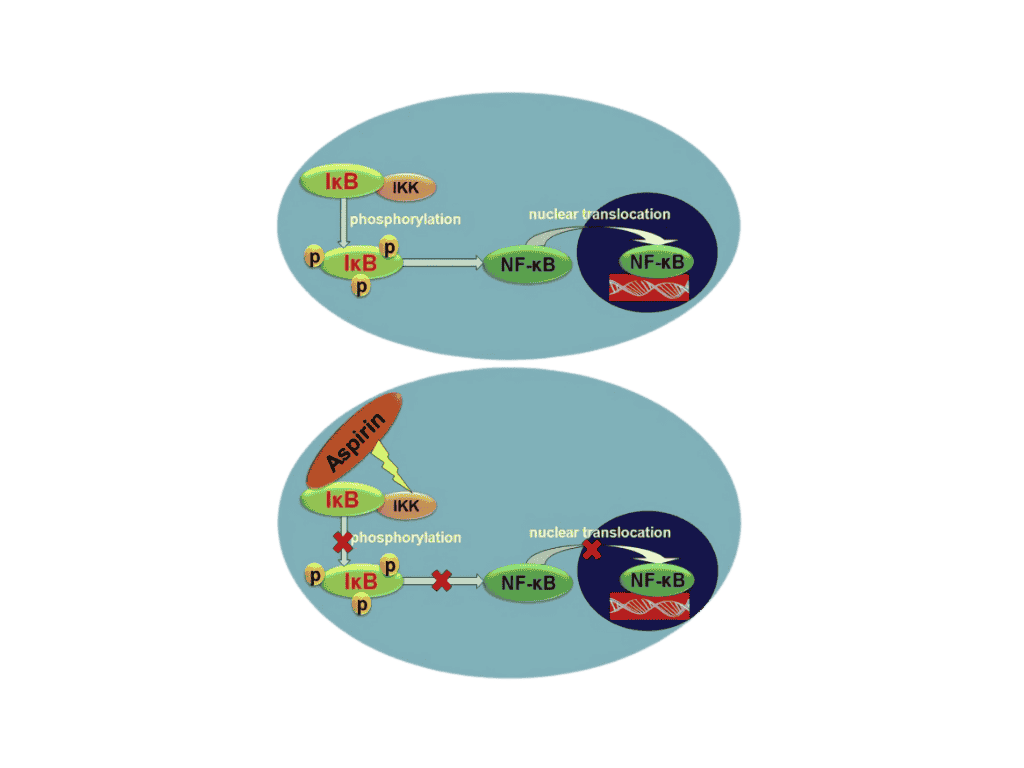
- Aspirin combines with IkB preventing its phosphorylated by IKK which ultimately prevents the translocation of NF-kB into the cell nucleus. Translocation of NF-kB into the nucleus promotes tumor growth.
Figure 2. Mechanism for Inhibition of NF-kB Translocation.
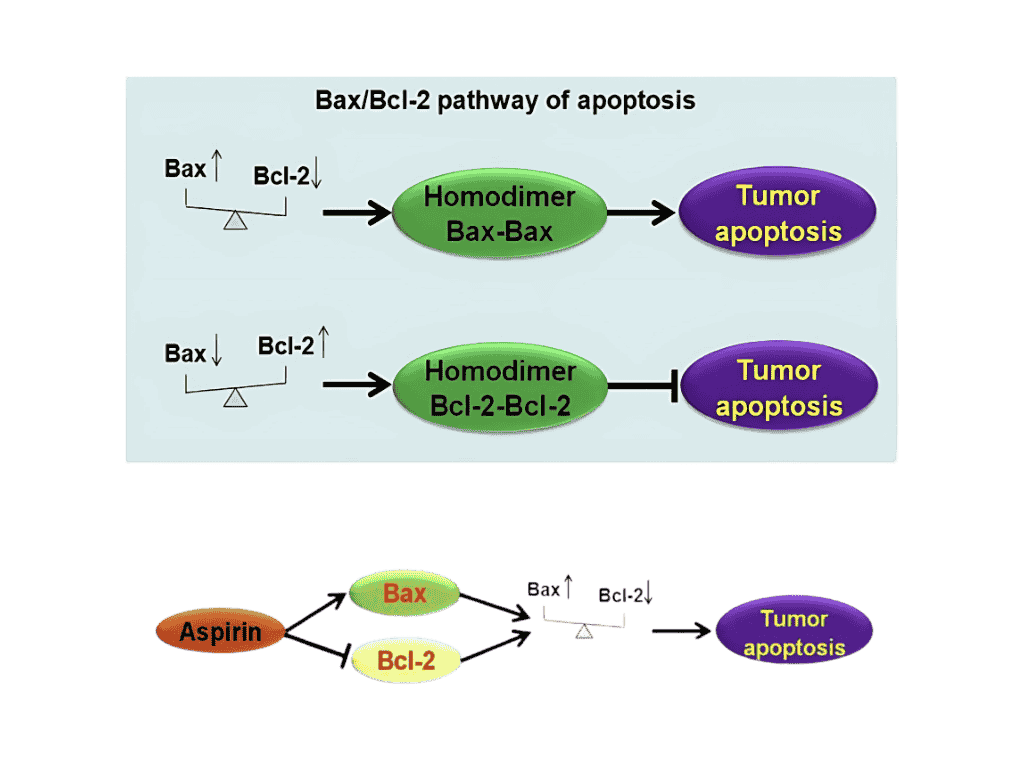
- Aspirin induces cancer cells to increase the amount of Bax protein and decrease the amount of Bcl-2 which favors cancer cell apoptosis (cell death).
Figure 3. Mechanism for Aspirin Induction of Cancer Cell Apoptosis.
Aspirin Inhibits Hypoxia-Mediated Lung Cancer Cell Stemness and Exosome Function by Chen Et Al.
a. When the lung cancer cell line A549 was incubated under hypoxic conditions to simulate an in vivo tumor environment incubation with aspirin resulted in the following changes
1. Aspirin inhibited stemness (stemness is a less differentiated cellular stated associated with tumor cells).
2. Inhibited cell growth by arresting the cell division cycle at the G2/M stage.
3. A hypoxic tumor environment induces tumor cells to release exosomes which enhance tumor progression. Aspirin reduced the number exosomes released and changed the contents of exosomes released.
“Tumor-Associated Macrophages Promote Lung Metastasis and Induce Epithelial Mesenchymal Transition in Osteosarcoma by activating the COX-2/STAT3 Axis”, by Han et al. Cancer Letters (2019).
a. Tumor-associated macrophages induce increased Cox-2 enzyme levels in cancer cells which supports tumor metastasis. Aspirin inhibits Cosx2.
b. Nude mice were inoculated with a cancer cell line and the amount of tumor metastasis to the lung was assessed by lung weight six weeks after inoculation for control mice and mice to which aspirin was added in drinking water (0.6 mg/mL). Lung weight (metastasis) was significantly reduced for the animals, receiving aspirin.
“Aspirin Ameliorates Lung Cancer by Targeting the miR-98/WNT1 Axis”, by Gan et al, Thoracic Cancer. (2019).
a. miR-98 acts as a gene expression regulator by down regulating expression of the protein WNT1 which supports cancer cell growth. In a lung cancer cell line (A549) aspirin increases the level of miR-98, reducing the level of WNT1 protein inhibiting cancer cell growth.
“Aspirin Inhibits Epithelial-to Mesenchymal Transition and Migration of Oncogenic K-ras Expressing Non-Small Cell Lung Carcinoma Cells by Down Regulating E-cadherin Repressor Slug”, by Khan et al. BMJ Cancer (2016)
a. Migratory potential (metastatic potential) of a lung cancer cell line (A549 cells) decreases upon incubation with aspirin through the following mechanism. Aspirin reduces the level of Slug protein, and the Slug protein reduces the level of E-cadherin. E-cadherin reduces the migratory potential of cancer cells. The net effect of aspirin inhibition of Slug is an increase in the level of E-cadherin and decreased migratory potential of lung cancer cells.
“Systematic Review Update of Observational Studies Further Supports Aspirin Role in Cancer Treatment: Time to Share Evidence and Decision-Making with Patients”, by Elwood et al. PLOS ONE (2018)
a. Subjects with cancer who received aspirin post-diagnosis had improved overall survival. The risk of mortality was reduced by 16% 28% and 31% for patients with lung, colorectal and breast cancer, respectively.
b. For all cancers the average risk of metastasis is reduced 21% for patients which receive aspirin post- diagnosis.
Inhalation for Lung Cancer
Chemotherapeutics by
“Pharmacokinetics Profile of Inhaled Submicron Particle Paclitaxel (NanoPac) in a Rodent Model”, by Verco et al. J Aerosol Med Pulm. Drug Deliv. 2019 Apr.
a. In a rat model lung exposure to the chemotherapeutic Paclitaxel was dramatically increased when the dose was delivered by an inhalation route (nebulization) relative to intravenous administration.
b. In a rat model systemic exposure to the chemotherapeutic Paclitaxel was dramatically reduced when the dose was delivered by inhalation (nebulization) relative to intravenous administration.
“Inhalation Delivery of Topotecan is Superior to Intravenous Exposure for Suppressing Lung Cancer in a Preclinical Model”, by Kuehl, Drug Deliv. (2018)
a. In a rat model lung exposure to the chemotherapeutic Topotecan was dramatically increased when the dose was delivered by an inhalation route (dry powder inhalation) relative to intravenous administration.
Non-clinical studies indicate that aspirin acts directly on lung cancer cells, inhibiting their growth, suggesting that topical delivery through inhalation may be more effective than oral administration.
Oral aspirin has already been linked to improved survival rates in cancer patients, and studies using animal models indicate that inhaled chemotherapeutics could further enhance survival outcomes in lung cancer therapy.